The aim is to detect potential bio-barrier toxicity, pro-inflammation and ciliopatic effect.
Apical or basolateral exposure studies can be run to mimic airborne or systemic delivery respectively.
The aim is to detect potential bio-barrier toxicity, pro-inflammation and ciliopatic effect.
Apical or basolateral exposure studies can be run to mimic airborne or systemic delivery respectively.

Example of a 90 days repeated dose exposure study on MucilAir™. 6 hours per day exposure to formaldehyde for a period of 90 days.
Every day, tissue integrity (TEER) was measured (N=3), then epithelia were reused for the next exposure.
Measurement of cytokines/chemokines or metalloproteinases: IL-8, IL-6, etc. release
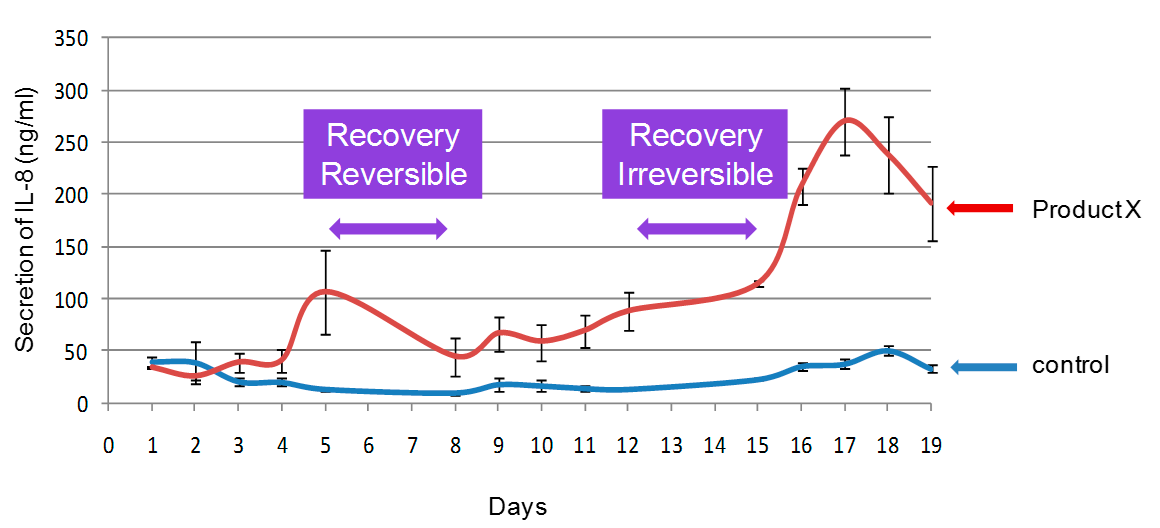
Example of Repeated Dose Exposure Study. Pro-inflammatory profiling is detected by measuring IL-8 release. Product X was applied twice per day for a period of 20 Days. Interestingly, reversible vs irreversible effects can be pointed out.
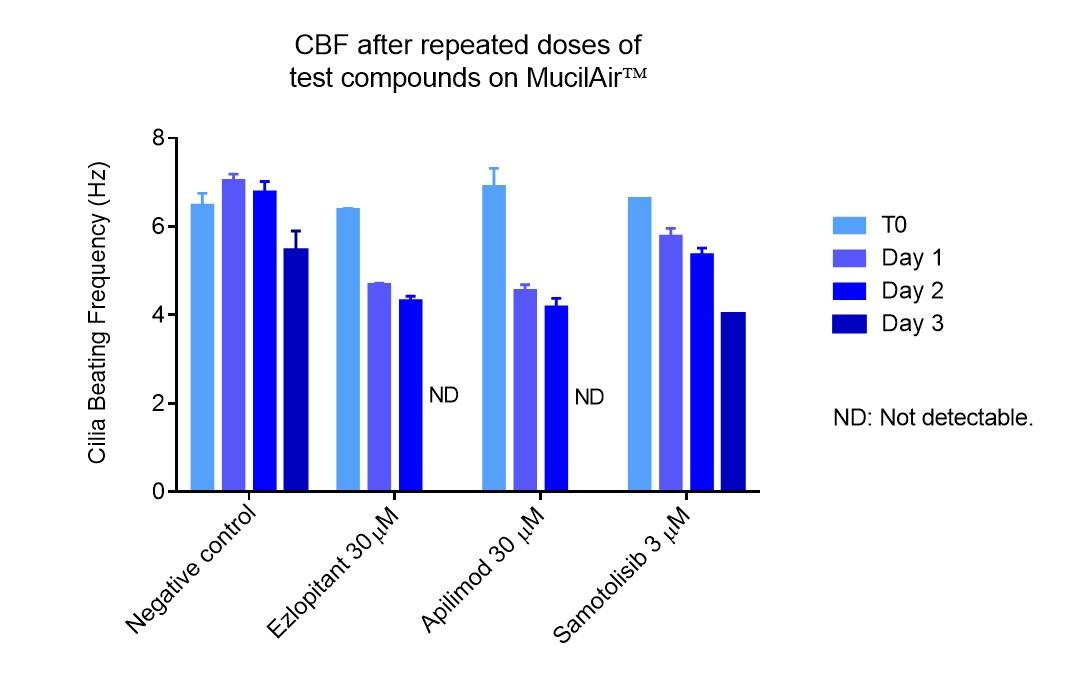
Example of ciliopathic effect after repeated dose basal exposure of APIs. Partial or total inhibition of Cilia Beating Frequency is easily monitored on MucilAir™ using Cilia-X.